Clinical Operations
The team at Zyfis Lifesciences have the necessary experience, expertise and global resources to effectively plan and manage your pivotal clinical trials keeping in mind projected timelines. Zyfis Lifesciences has partnered with some of the largest pharmaceuticals, biotech and device companies, providing innovative and customized clinical trial and research services.
Till date, as a team we have completed several large-scale Phase I, Phase II and Phase III trials successfully by implementing complete clinical trial management oversight, medical monitoring, data management and site management services executing high quality program in accordance with Good Clinical Practices (GCP) and regulatory compliance with a focus on clinical trials in India. From start to end, we’ll ensure that all aspects of your trial are professionally handled with quality and integrity of data always at the forefront while establishing the shortest route to market for your product.
Project Management
Project Management is our major competence. Zyfis owns a set-up, that is grounded upon project managers and skilled investigators with enormous experience who have the ability to efficiently lead projects from the start till end. Zyfis Project Managers have extensive experience in the conducting complex clinical trials, which is essential to study success. Project Planning, Training and Kick-off :
➩ Organizing and conducting comprehensive project kick-off meeting with study team, Project design development, Development of Protocol and other training materials and conduct training sessions. Project Oversight and Reporting :
➩ Manage the project timelines and budget. Track study activity on weekly/biweekly basis and reporting throughout project duration to the sponsor. Vendor Management :
➩ Zyfis performs Vendor selection, qualification, contracting, and takes care of payment administration. End-to-end management of critical study vendors (e.g. drug depot, lab, IRB/EC, eTMF, EDC & RTSM (Randomization and Trial Supply Management)

Regulatory Affairs
Our Regulatory team provides comprehensive support for Sponsors/Clients to overcome the hurdles linked with the requirements of the DCGI, FDA, EMA and Other Regulatory Bodies. We have a remarkable track record of getting on time approvals for clinical trials, from different regulatory jurisdiction. We help our Clients to understand the requirement of respective Regulatory Authority and assist them appropriately to avoid any delay in approval. We cater to our Sponsors/Clients in end to end process in a timely manner so that their product can go out in the market as scheduled.
Compilation of Regulatory Packages:
➩ Preparing regulatory package for Regulatory Authorities. Coordinating and submitting Protocol or Protocol Amendments. Submitting interim and annual reports.Communication with Regulatory Authority
➩ Communication and follow up with competent authorities including DCGI, FDA and EMA. Study and Investigator submissions to Ethics Committees and IRBs. Study Registration on respective registries, and other trial databases.Import/Export Management
➩ Management of any required import/export activities for IP and other study materials and samples. Management of regulated products.Data Management
Zyfis provides comprehensive data management services for all phases of the clinical trial, emphasizing quality control and state-of-the-art technology. We provide end to end data management services (data processing, analysis, and management) via our expert personnel including bioinformatics specialists – researchers that are proficient in clinical software tools. Accuracy and integrity are reinforced with timed quality assurance procedures and timed status reports, according to the project’s requirements.
Our Data management services include
⇒ Data quality management⇒ Querying the data for completion
⇒ Assistance in writing the interim and final reports
⇒ Data validation and analysis
⇒ Creation of study reports
⇒ Drafting of CRFs
⇒ Quality control of data


Medical Writing
We have dedicated medical team of medical writers having long track records in scientific and/or clinical research areas, ensuring that all medical writing requirements (DCGI, EMA, FDA etc.) are met in an efficient and timely manner.
Medical writing & safety We have dedicated medical team of medical writers having long track records in scientific and/or clinical research areas, ensuring that all medical writing requirements (DCGI, EMA, FDA etc.) are met in an efficient and timely manner.
⇒ Protocol conceptualization, designing and preparation
⇒ ICF designing
⇒ Preparation of abstracts and research and/or review articles, short communications etc.
⇒ Preparation of Posters
⇒ Report writing in compliance with ICH E3 guidelines/applicable regulatory submission
⇒ Site specific and country specific reports
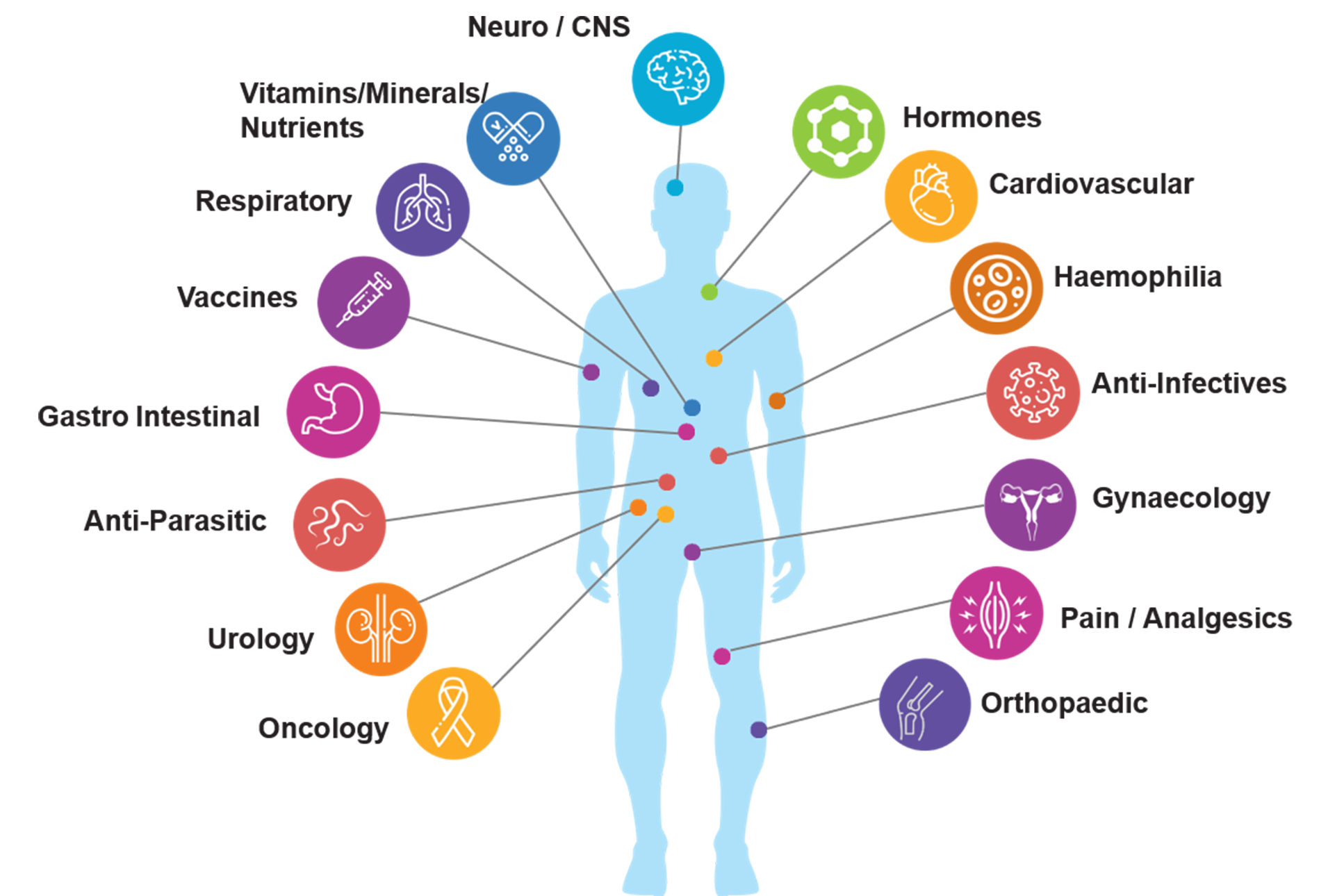
⇒ Therapeutic Experience
Our diverse platform covers
⇒ Psychiatry
⇒ Cardiology
⇒ Nephrology
⇒ Ophthalmology
⇒ Vaccine
⇒ Pain Management specialties
Biostatistics & Statistical Programming
➩ Development of study design and study protocols
➩ Definition of study objectives and endpoints
➩ Sample size determination and randomization plan
➩ Preparation of statistical analysis plans, consistent with ICH guidelines
➩ SAS programming
➩ Statistical analyses and reporting of study results
➩ Production of tables, listings and graphs, in compliance with ICH guidelines
➩ Validated software: SAS® 9.2 and WinNonlin 5.0.1 for apt output
➩ Exhaustive discrepancy management
➩ Protocol writing
➩ Tracking protocol deviations
➩ Database creation and data lockup


Therapeutic Expertise
➩ Respiratory
➩ Cardiology
➩ Gynaecology
➩ Ophthalmology
➩ Neurology/psychiatry
DATA MANAGEMENT & SOLUTIONS
DATA SOLUTIONS
Pharma Covigilance
Zyfis PV is a software solution that enables complex data analysis and querying of safety datasets for the pharma industry. It meets all the global regulatory requirements and ensures high quality standards and compliance.